How Does Changing The Electronegativity Affect Bond Polarity
i.9: Electronegativity and Bail Polarity (Review)
- Page ID
- 136796
Learning Objective
- Identify polar bonds and compounds
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the virtually electronegative chemical element) is assigned a value of 4.0, and values range downwardly to cesium and francium which are the least electronegative at 0.7.
Patterns of electronegativity in the Periodic Tabular array
Electronegativity is defined as the ability of an atom in a detail molecule to attract electrons to itself. The greater the value, the greater the bewitchery for electrons.
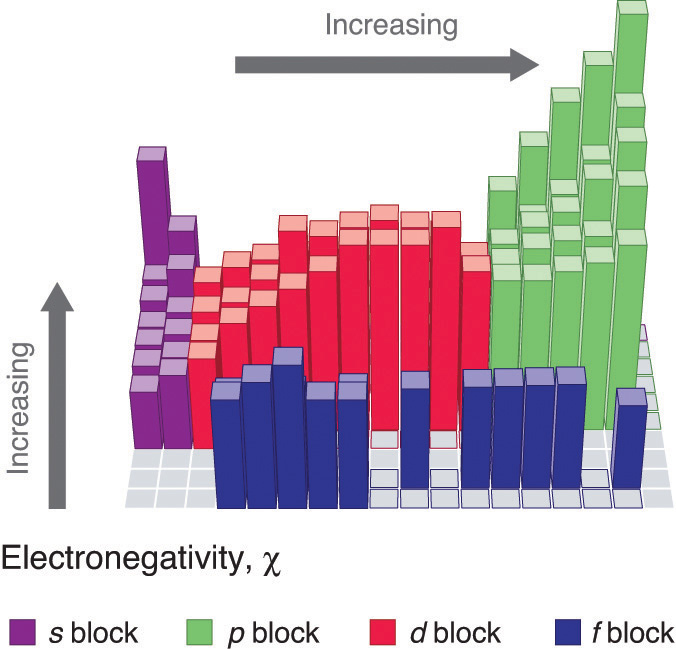
Trends in electronegativity across a period
The positively charged protons in the nucleus concenter the negatively charged electrons. Equally the number of protons in the nucleus increases, the electronegativity or attraction volition increment. Therefore electronegativity increases from left to correct in a row in the periodic table. This upshot just holds true for a row in the periodic table because the attraction between charges falls off rapidly with distance. The chart shows electronegativities from sodium to chlorine (ignoring argon since it does not does not form bonds).
Trends in electronegativity down a group
Every bit you go downward a group, electronegativity decreases. (If it increases up to fluorine, it must subtract as you become down.) The chart shows the patterns of electronegativity in Groups 1 and 7.
Explaining the patterns in electronegativity
The attraction that a bonding pair of electrons feels for a particular nucleus depends on:
- the number of protons in the nucleus;
- the distance from the nucleus;
- the amount of screening by inner electrons.
Why does electronegativity increment across a menstruum?
Consider sodium at the beginning of period 3 and chlorine at the finish (ignoring the noble gas, argon). Think of sodium chloride as if it were covalently bonded.
Both sodium and chlorine have their bonding electrons in the 3-level. The electron pair is screened from both nuclei by the 1s, 2s and 2p electrons, but the chlorine nucleus has 6 more protons in it. Information technology is no wonder the electron pair gets dragged and then far towards the chlorine that ions are formed. Electronegativity increases across a period considering the number of charges on the nucleus increases. That attracts the bonding pair of electrons more strongly.
Why does electronegativity autumn as you lot become down a grouping?
Equally you go downward a grouping, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Consider the hydrogen fluoride and hydrogen chloride molecules:
The bonding pair is shielded from the fluorine's nucleus only by the 1s2 electrons. In the chlorine case it is shielded by all the 1s22s22p6 electrons. In each case there is a net pull from the eye of the fluorine or chlorine of +7. But fluorine has the bonding pair in the ii-level rather than the 3-level as it is in chlorine. If information technology is closer to the nucleus, the allure is greater.
Dipole moments occur when at that place is a separation of charge. They tin can occur between two ions in an ionic bond or between atoms in a covalent bond; dipole moments arise from differences in electronegativity. The larger the difference in electronegativity, the larger the dipole moment. The distance between the charge separation is also a deciding cistron into the size of the dipole moment. The dipole moment is a measure out of the polarity of the molecule.
Bond Polarity & Dipole Moment
Atoms with differences in electronegativity will share electrons unequally. The shared electrons of the covalent bail are held more tightly at the more electronegative element creating a partial negative charge, while the less electronegative element has a fractional positive charge, . The larger the difference in electronegativity between the two atoms, the more polar the bail. To be considered a polar bond, the departure in electronegativity must >0.4 on the Pauling scale. Since the 2 electrical fractional charges have reverse sign and equal magnitude and are separated past a distance, a dipole is established. Dipole moment is measured in debye units, which is equal to the distance between the charges multiplied by the charge (1 debye equals 3.34 x 10 -xxx coulomb-meters).
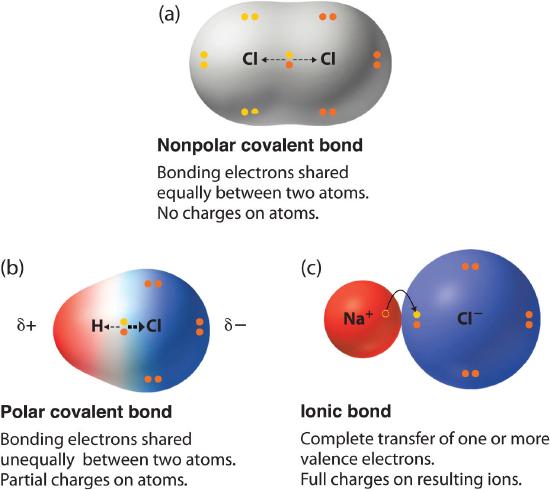
Polarity and Structure of Molecules
The shape of a molecule AND the polarity of its bonds. A molecule that contains polar bonds, might non have any overall polarity, depending upon its shape. The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. If these centers prevarication at the aforementioned point in space, so the molecule has no overall polarity (and is not polar).
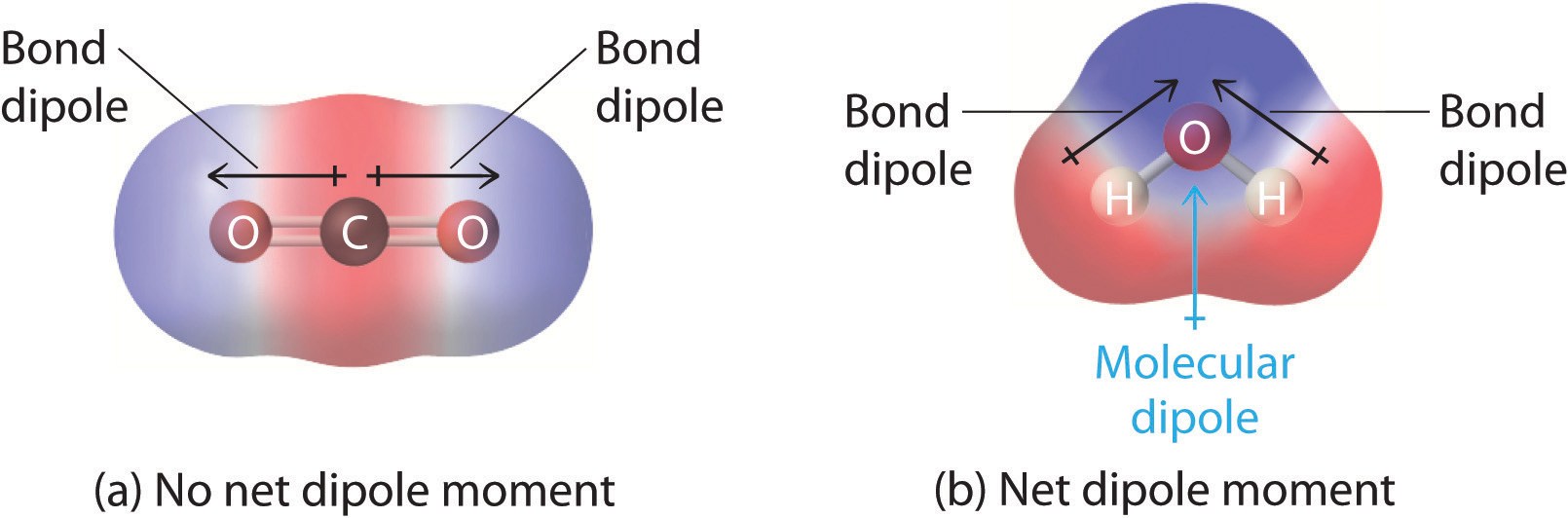
If a molecule is completely symmetric, then the dipole moment vectors on each molecule will abolish each other out, making the molecule nonpolar. A molecule can only be polar if the structure of that molecule is non symmetric.
A good example of a nonpolar molecule that contains polar bonds is carbon dioxide. This is a linear molecule and the C=O bonds are, in fact, polar. The central carbon will have a cyberspace positive charge, and the two outer oxygens a internet negative charge. However, since the molecule is linear, these 2 bond dipoles cancel each other out (i.due east. vector addition of the dipoles equals nil). And the overall molecule has no dipole ( μ = 0 .
Although a polar bond is a prerequisite for a molecule to have a dipole, not all molecules with polar bonds exhibit dipoles
Geometric Considerations
How Does Changing The Electronegativity Affect Bond Polarity,
Source: https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_Chem_420_-_Organic_Chemistry_I/Text/01%3A_Introduction_and_Review/1.09%3A_Electronegativity_and_Bond_Polarity_(Review)#:~:text=The%20shared%20electrons%20of%20the,the%20more%20polar%20the%20bond.
Posted by: lamoureuxtheatanthe.blogspot.com


0 Response to "How Does Changing The Electronegativity Affect Bond Polarity"
Post a Comment